Drug-eluting Stent
Introduction
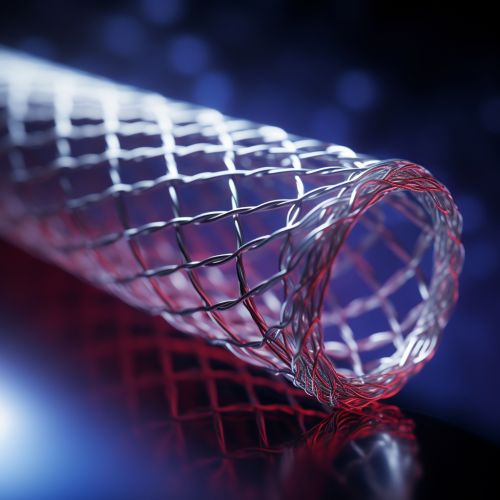
A drug-eluting stent (DES) is a coronary stent typically used in the treatment of coronary artery disease (CAD). It is a small, expandable tube made of medical-grade stainless steel or cobalt alloy metal. The stent is coated with medication that is slowly released into the artery following placement. The primary function of a DES is to keep the patient's artery open and prevent restenosis, or the re-narrowing of the artery, after an angioplasty procedure.
History
The concept of the drug-eluting stent was first introduced in the late 1990s, following the widespread use of bare-metal stents (BMS). While BMS were effective in treating CAD, they had a significant drawback: a high rate of restenosis. This led to the development of DES, which were designed to reduce this risk by releasing a drug that prevents cell proliferation.
Design and Construction
A drug-eluting stent is composed of three main components: the stent platform, the drug, and the polymer coating. The stent platform is typically made of a metallic alloy, which provides the necessary structural integrity to keep the artery open. The drug, which is embedded in the polymer coating, is gradually released into the arterial wall to prevent restenosis.
Mechanism of Action
The primary mechanism of action of a drug-eluting stent is the prevention of restenosis. This is achieved through the gradual release of a drug from the stent's polymer coating into the arterial wall. The drug inhibits the proliferation of smooth muscle cells, which is a primary cause of restenosis following stent placement.
Clinical Use
Drug-eluting stents are primarily used in the treatment of coronary artery disease. They are placed in the artery during a procedure known as percutaneous coronary intervention (PCI), also commonly referred to as angioplasty with stent placement. DES have been shown to significantly reduce the risk of restenosis compared to bare-metal stents.
Efficacy and Safety
Numerous clinical trials have demonstrated the efficacy and safety of drug-eluting stents. They have been shown to significantly reduce the risk of restenosis compared to bare-metal stents. However, like all medical devices, DES have potential risks and complications, including stent thrombosis.
Future Developments
Research is ongoing into the development of new types of drug-eluting stents, including bioabsorbable stents and stents with different drug and polymer combinations. These developments aim to further improve the efficacy and safety of DES.
