X-ray emission spectroscopy
Introduction
X-ray emission spectroscopy (XES) is a technique used in physics, chemistry, and materials science to study the electronic structure of materials. This method involves the measurement of X-ray photons emitted by a material after it has been excited by incident X-rays. The energy and intensity of the emitted X-rays provide valuable information about the atomic, electronic, and chemical structure of the material under study.
Principles of X-ray Emission Spectroscopy
XES is based on the principle of fluorescence, where an atom absorbs energy and re-emits it in the form of light. In the case of XES, the absorbed energy is in the form of X-ray photons. When an X-ray photon is absorbed by an atom, it can cause an inner-shell electron to be ejected from the atom. This creates a vacancy in the electron shell, which is then filled by an electron from a higher energy level. The energy difference between the two levels is released in the form of an X-ray photon, which is detected and measured in the XES experiment.
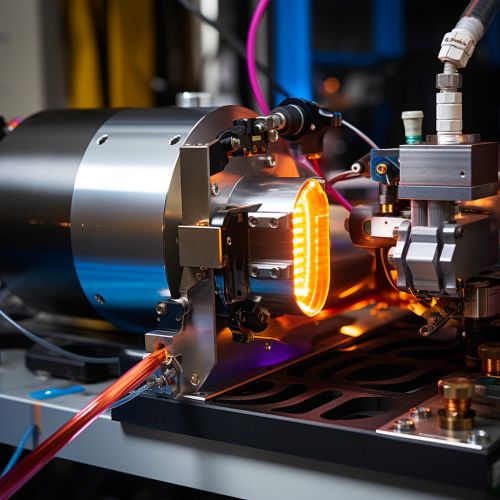

Experimental Setup
The experimental setup for XES typically includes an X-ray source, a sample holder, and a detector. The X-ray source generates the incident X-rays that are used to excite the sample. The sample holder positions the sample in the path of the incident X-rays. The detector measures the energy and intensity of the X-rays emitted by the sample. The data collected by the detector is then analyzed to determine the electronic structure of the sample.
Applications of X-ray Emission Spectroscopy
XES has a wide range of applications in various fields of science and technology. In physics and materials science, it is used to study the electronic structure of materials, including semiconductors, superconductors, and magnetic materials. In chemistry, it is used to investigate the electronic structure of molecules, including their bonding and electronic transitions. In environmental science, it is used to analyze the chemical composition of soils, rocks, and water samples. In archaeology, it is used to determine the composition of ancient artifacts and materials.
Advantages and Limitations
One of the main advantages of XES is its ability to provide detailed information about the electronic structure of materials. It can probe both the occupied and unoccupied electronic states, making it a powerful tool for studying a wide range of materials. It is also a non-destructive technique, meaning that the sample is not damaged during the experiment.
However, XES also has some limitations. The main limitation is that it requires a high-intensity X-ray source, which is not readily available in most laboratories. It also requires a high-resolution detector to accurately measure the energy of the emitted X-rays. Furthermore, the interpretation of XES spectra can be complex, requiring sophisticated computational methods and models.
