Bacterial Communication and Quorum Sensing in Science
Introduction
Bacterial communication and quorum sensing represent a sophisticated form of interaction among bacterial populations, enabling them to coordinate behavior in response to environmental stimuli. This phenomenon is crucial for understanding bacterial ecology, pathogenesis, and the development of novel therapeutic strategies. The study of quorum sensing has revealed complex regulatory networks that control a variety of bacterial functions, including virulence, biofilm formation, and antibiotic resistance.
Mechanisms of Quorum Sensing
Quorum sensing is a cell-density-dependent signaling mechanism that bacteria use to regulate gene expression. This process involves the production, release, and detection of chemical signal molecules known as autoinducers. As the bacterial population grows, the concentration of autoinducers increases, allowing bacteria to sense their population density and coordinate collective behaviors.
Autoinducers
Autoinducers are small signaling molecules that vary among bacterial species. Gram-negative bacteria typically use acyl-homoserine lactones (AHLs), while Gram-positive bacteria often use oligopeptides. A third type, autoinducer-2 (AI-2), is used by both Gram-negative and Gram-positive bacteria, suggesting a universal signaling mechanism.
Signal Transduction Pathways
Upon reaching a threshold concentration, autoinducers bind to specific receptor proteins, triggering a signal transduction cascade that alters gene expression. In Gram-negative bacteria, this often involves the LuxI/LuxR system, where LuxI synthesizes AHLs and LuxR acts as a receptor. In Gram-positive bacteria, two-component systems involving histidine kinases and response regulators are common.
Functions of Quorum Sensing
Quorum sensing regulates a wide array of bacterial behaviors, many of which are critical for survival and adaptation.
Biofilm Formation
Biofilms are structured communities of bacteria encased in a self-produced extracellular matrix. Quorum sensing plays a pivotal role in biofilm development by regulating the expression of genes involved in adhesion, matrix production, and community architecture. This process enhances bacterial resistance to environmental stresses and antibiotics.
Virulence Factor Production
In pathogenic bacteria, quorum sensing controls the expression of virulence factors, which are molecules that facilitate infection and disease progression. For example, Pseudomonas aeruginosa, a common opportunistic pathogen, uses quorum sensing to regulate the production of toxins and enzymes that damage host tissues.
Antibiotic Resistance
Quorum sensing contributes to antibiotic resistance by regulating genes involved in efflux pumps and biofilm formation. Biofilms act as a physical barrier to antibiotics, while efflux pumps actively expel antibiotics from bacterial cells, reducing their efficacy.
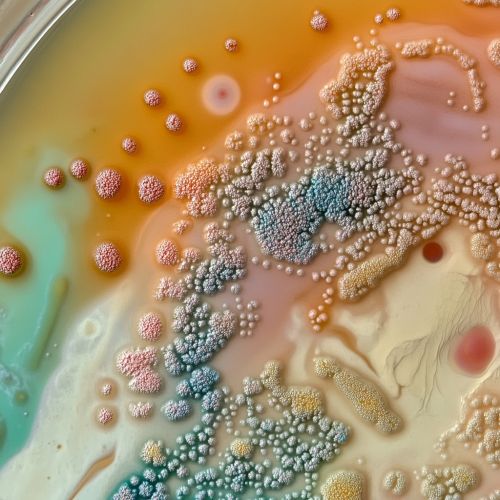

Quorum Sensing in Different Bacterial Species
The mechanisms and functions of quorum sensing can vary significantly among different bacterial species.
Vibrio fischeri
Vibrio fischeri is a model organism for studying quorum sensing. It forms a symbiotic relationship with the Hawaiian bobtail squid, using quorum sensing to regulate bioluminescence. This process is mediated by the LuxI/LuxR system, where AHLs produced by LuxI activate LuxR, leading to the expression of bioluminescent genes.
Staphylococcus aureus
Staphylococcus aureus uses a quorum sensing system known as the accessory gene regulator (agr) system. This system controls the expression of virulence factors and is critical for the pathogenesis of infections such as skin abscesses and pneumonia.
Escherichia coli
In Escherichia coli, quorum sensing is involved in the regulation of biofilm formation and virulence. The AI-2 signaling system is particularly important, allowing E. coli to communicate with other bacterial species in mixed communities.
Quorum Sensing Inhibition
Given its role in pathogenesis, quorum sensing is an attractive target for novel antimicrobial therapies. Quorum sensing inhibitors (QSIs) are compounds that disrupt quorum sensing pathways, potentially reducing bacterial virulence and enhancing the efficacy of traditional antibiotics.
Natural QSIs
Several natural compounds have been identified as QSIs, including furanones from marine algae and garlic-derived ajoene. These compounds interfere with autoinducer synthesis or receptor binding, disrupting quorum sensing-mediated behaviors.
Synthetic QSIs
Synthetic QSIs are being developed to target specific quorum sensing pathways. These compounds are designed to be more potent and selective than natural QSIs, offering a promising approach to combat antibiotic-resistant infections.
Applications and Implications
Understanding quorum sensing has significant implications for various fields, including medicine, agriculture, and biotechnology.
Medical Applications
In medicine, targeting quorum sensing could lead to new treatments for bacterial infections, particularly those involving biofilms or antibiotic-resistant strains. QSIs could be used in combination with antibiotics to enhance their effectiveness.
Agricultural Applications
In agriculture, quorum sensing research can inform the development of strategies to control plant pathogens and promote beneficial plant-microbe interactions. This could lead to more sustainable agricultural practices and improved crop yields.
Biotechnological Applications
In biotechnology, quorum sensing can be harnessed for the production of valuable compounds, such as antibiotics and enzymes. By manipulating quorum sensing pathways, researchers can optimize microbial production processes.
