Osteoblasts: Difference between revisions
(Created page with "== Introduction == Osteoblasts are specialized cells responsible for the synthesis and mineralization of bone during both initial bone formation and later bone remodeling. These cells play a crucial role in the maintenance, growth, and repair of the skeletal system. Osteoblasts originate from mesenchymal stem cells and differentiate through a series of stages, ultimately becoming mature bone-forming cells. Understanding the function and regulation of osteoblasts is esse...") |
No edit summary |
||
| Line 40: | Line 40: | ||
The mineralization process involves the deposition of hydroxyapatite crystals within the osteoid. Osteoblasts regulate this process by controlling the local concentration of calcium and phosphate ions, as well as by secreting matrix vesicles that serve as nucleation sites for hydroxyapatite formation. | The mineralization process involves the deposition of hydroxyapatite crystals within the osteoid. Osteoblasts regulate this process by controlling the local concentration of calcium and phosphate ions, as well as by secreting matrix vesicles that serve as nucleation sites for hydroxyapatite formation. | ||
[[Image:Detail-91529.jpg|thumb|center|Microscopic image of osteoblasts forming bone tissue.|class=only_on_mobile]] | |||
[[Image:Detail-91530.jpg|thumb|center|Microscopic image of osteoblasts forming bone tissue.|class=only_on_desktop]] | |||
== Role in Bone Remodeling == | == Role in Bone Remodeling == | ||
Latest revision as of 20:41, 20 June 2024
Introduction
Osteoblasts are specialized cells responsible for the synthesis and mineralization of bone during both initial bone formation and later bone remodeling. These cells play a crucial role in the maintenance, growth, and repair of the skeletal system. Osteoblasts originate from mesenchymal stem cells and differentiate through a series of stages, ultimately becoming mature bone-forming cells. Understanding the function and regulation of osteoblasts is essential for comprehending various bone-related diseases and conditions, such as osteoporosis and fractures.
Origin and Differentiation
Osteoblasts are derived from mesenchymal stem cells (MSCs), which are multipotent stromal cells capable of differentiating into various cell types, including osteoblasts, chondrocytes, myocytes, and adipocytes. The differentiation process of osteoblasts involves several stages:
1. **Proliferation**: MSCs proliferate and commit to the osteoblast lineage. 2. **Pre-osteoblasts**: These committed cells express early markers such as Runx2 and Osterix. 3. **Mature Osteoblasts**: These cells express markers like alkaline phosphatase (ALP), collagen type I, and osteocalcin. 4. **Osteocytes**: Some osteoblasts become embedded in the bone matrix and differentiate into osteocytes, which are crucial for bone maintenance.
Molecular Regulation
The differentiation and activity of osteoblasts are tightly regulated by various signaling pathways and transcription factors:
Wnt/β-catenin Signaling
The Wnt signaling pathway is critical for osteoblast differentiation and function. Activation of Wnt signaling leads to the stabilization and accumulation of β-catenin in the cytoplasm, which then translocates to the nucleus to activate the transcription of osteogenic genes.
Bone Morphogenetic Proteins (BMPs)
Bone morphogenetic proteins are a group of growth factors known to play a significant role in bone formation. BMPs bind to their receptors on the cell surface, initiating a signaling cascade that promotes the expression of osteogenic genes.
Runx2 and Osterix
Runx2 and Osterix are essential transcription factors for osteoblast differentiation. Runx2 is required for the early stages of osteoblast differentiation, while Osterix is necessary for the maturation of osteoblasts.
Function
Osteoblasts are primarily responsible for the formation of new bone tissue. They secrete a variety of extracellular matrix proteins, including collagen type I, which forms the scaffold for bone mineralization. Osteoblasts also produce enzymes such as alkaline phosphatase, which hydrolyzes phosphate esters, providing inorganic phosphate for hydroxyapatite formation.
Bone Matrix Production
Osteoblasts synthesize and secrete the organic components of the bone matrix, including collagen type I, osteocalcin, osteopontin, and bone sialoprotein. These proteins form the osteoid, an unmineralized bone matrix that later undergoes mineralization.
Mineralization
The mineralization process involves the deposition of hydroxyapatite crystals within the osteoid. Osteoblasts regulate this process by controlling the local concentration of calcium and phosphate ions, as well as by secreting matrix vesicles that serve as nucleation sites for hydroxyapatite formation.

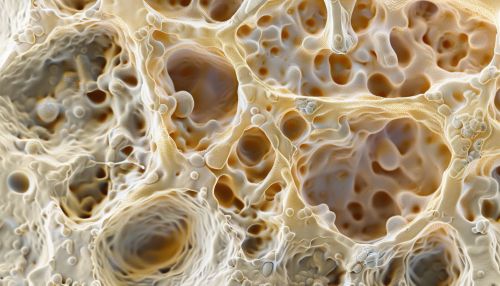
Role in Bone Remodeling
Bone remodeling is a continuous process involving the resorption of old bone by osteoclasts and the formation of new bone by osteoblasts. This process is essential for maintaining bone strength and integrity, as well as for the repair of micro-damage.
Coupling of Bone Resorption and Formation
The activities of osteoclasts and osteoblasts are tightly coupled. Osteoclasts create resorption pits in the bone matrix, which are subsequently filled by osteoblasts with new bone tissue. This coupling is mediated by various signaling molecules, including RANKL, OPG, and M-CSF.
Mechanical Loading
Mechanical loading and physical activity stimulate osteoblast activity and bone formation. Osteocytes, which are former osteoblasts embedded in the bone matrix, act as mechanosensors and transduce mechanical signals to osteoblasts, promoting bone formation in response to mechanical stress.
Pathological Conditions
Dysregulation of osteoblast function can lead to various bone diseases and conditions:
Osteoporosis
Osteoporosis is characterized by decreased bone mass and increased fracture risk. It results from an imbalance between bone resorption and formation, often due to reduced osteoblast activity or increased osteoclast activity.
Osteogenesis Imperfecta
Osteogenesis imperfecta is a genetic disorder caused by mutations in genes encoding collagen type I, leading to brittle bones and frequent fractures. Osteoblasts in individuals with this condition produce defective collagen, impairing bone strength.
Paget's Disease of Bone
Paget's disease of bone involves abnormal bone remodeling, with excessive bone resorption followed by disorganized bone formation. Osteoblasts in affected individuals produce woven bone instead of the normal lamellar bone, resulting in structurally weak bones.
Therapeutic Approaches
Understanding osteoblast biology has led to the development of various therapeutic strategies for bone-related diseases:
Anabolic Agents
Anabolic agents, such as teriparatide (a recombinant form of parathyroid hormone), stimulate osteoblast activity and bone formation. These agents are used in the treatment of osteoporosis to increase bone mass and reduce fracture risk.
Anti-resorptive Agents
Anti-resorptive agents, such as bisphosphonates and denosumab, inhibit osteoclast activity, thereby reducing bone resorption. These agents indirectly benefit osteoblasts by maintaining bone mass and structural integrity.
Gene Therapy
Gene therapy approaches aim to enhance osteoblast function by delivering genes encoding osteogenic factors, such as BMPs or Runx2, to target cells. This strategy holds promise for treating genetic bone disorders and enhancing bone regeneration.
Research and Future Directions
Ongoing research aims to further elucidate the molecular mechanisms regulating osteoblast function and to develop novel therapeutic approaches for bone diseases. Areas of interest include:
Stem Cell Therapy
Stem cell therapy involves the use of MSCs or induced pluripotent stem cells (iPSCs) to regenerate bone tissue. Researchers are exploring ways to enhance the osteogenic potential of these cells for use in bone repair and regeneration.
Biomaterials and Scaffolds
The development of biomaterials and scaffolds that mimic the natural bone environment is a promising area of research. These materials can support osteoblast attachment, proliferation, and differentiation, facilitating bone regeneration in clinical applications.
Epigenetic Regulation
Epigenetic modifications, such as DNA methylation and histone acetylation, play a role in the regulation of osteoblast differentiation and function. Understanding these modifications may lead to new therapeutic targets for bone diseases.
Conclusion
Osteoblasts are essential for bone formation, maintenance, and repair. Their function is regulated by a complex network of signaling pathways and transcription factors. Dysregulation of osteoblast activity can lead to various bone diseases, highlighting the importance of understanding osteoblast biology for developing effective therapies. Ongoing research continues to uncover new insights into the molecular mechanisms governing osteoblast function and holds promise for advancing the treatment of bone-related conditions.
See Also
- Osteoclast
- Bone remodeling
- Mesenchymal stem cell
- Wnt signaling pathway
- Bone morphogenetic protein
- Osteogenesis imperfecta
- Paget's disease of bone
