Osteocytes: Difference between revisions
(Created page with "== Introduction == Osteocytes are mature bone cells that originate from osteoblasts, which become embedded in the mineralized matrix of bone tissue. These cells play a crucial role in the maintenance, repair, and metabolism of bones. Osteocytes are the most abundant cells in bone tissue, comprising about 90-95% of all bone cells. They reside in small cavities called lacunae and extend their long cytoplasmic processes through tiny channels known as canaliculi, forming an...") |
No edit summary |
||
| Line 7: | Line 7: | ||
Osteocytes are characterized by their unique morphology and strategic positioning within the bone matrix. Each osteocyte is housed in a lacuna, a small cavity within the mineralized bone matrix. The lacunae are interconnected by canaliculi, which are microscopic channels that allow the osteocytes to communicate with each other and with cells on the bone surface. | Osteocytes are characterized by their unique morphology and strategic positioning within the bone matrix. Each osteocyte is housed in a lacuna, a small cavity within the mineralized bone matrix. The lacunae are interconnected by canaliculi, which are microscopic channels that allow the osteocytes to communicate with each other and with cells on the bone surface. | ||
[[Image:Detail-91947.jpg|thumb|center|Microscopic image of an osteocyte within its lacuna, showing the network of canaliculi.|class=only_on_mobile]] | |||
[[Image:Detail-91948.jpg|thumb|center|Microscopic image of an osteocyte within its lacuna, showing the network of canaliculi.|class=only_on_desktop]] | |||
The cytoplasmic processes of osteocytes extend through the canaliculi, forming a complex network that facilitates the exchange of nutrients, waste products, and signaling molecules. This network is essential for maintaining the viability and function of osteocytes, as well as for coordinating bone remodeling activities. | The cytoplasmic processes of osteocytes extend through the canaliculi, forming a complex network that facilitates the exchange of nutrients, waste products, and signaling molecules. This network is essential for maintaining the viability and function of osteocytes, as well as for coordinating bone remodeling activities. | ||
Latest revision as of 22:01, 18 June 2024
Introduction
Osteocytes are mature bone cells that originate from osteoblasts, which become embedded in the mineralized matrix of bone tissue. These cells play a crucial role in the maintenance, repair, and metabolism of bones. Osteocytes are the most abundant cells in bone tissue, comprising about 90-95% of all bone cells. They reside in small cavities called lacunae and extend their long cytoplasmic processes through tiny channels known as canaliculi, forming an extensive network that facilitates communication and nutrient exchange.
Structure and Location
Osteocytes are characterized by their unique morphology and strategic positioning within the bone matrix. Each osteocyte is housed in a lacuna, a small cavity within the mineralized bone matrix. The lacunae are interconnected by canaliculi, which are microscopic channels that allow the osteocytes to communicate with each other and with cells on the bone surface.
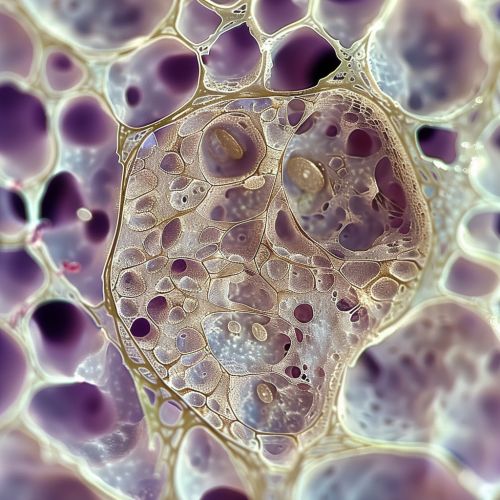
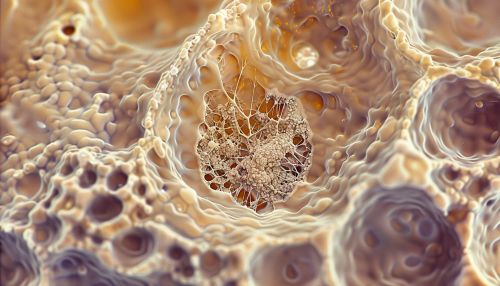
The cytoplasmic processes of osteocytes extend through the canaliculi, forming a complex network that facilitates the exchange of nutrients, waste products, and signaling molecules. This network is essential for maintaining the viability and function of osteocytes, as well as for coordinating bone remodeling activities.
Function
Osteocytes have several critical functions in bone biology, including:
Mechanosensation
Osteocytes act as mechanosensors, detecting mechanical strain and stress on the bone. When mechanical load is applied to the bone, osteocytes sense the deformation and translate it into biochemical signals. These signals are crucial for the regulation of bone remodeling, ensuring that bone formation and resorption are balanced according to mechanical demands.
Regulation of Bone Remodeling
Osteocytes play a key role in the regulation of bone remodeling by coordinating the activities of osteoblasts and osteoclasts. They secrete signaling molecules such as sclerostin, which inhibits bone formation by osteoblasts, and receptor activator of nuclear factor kappa-Β ligand (RANKL), which promotes the differentiation and activity of osteoclasts. Through these mechanisms, osteocytes help maintain bone homeostasis and adapt bone structure to mechanical needs.
Mineral Homeostasis
Osteocytes are involved in the regulation of mineral homeostasis, particularly calcium and phosphate. They can mobilize calcium from the bone matrix through a process called osteocytic osteolysis, which is important for maintaining serum calcium levels. Additionally, osteocytes produce factors that influence the mineralization of the bone matrix, ensuring proper bone density and strength.
Development and Differentiation
Osteocytes originate from osteoblasts, which are bone-forming cells. During bone formation, some osteoblasts become trapped within the mineralized matrix and differentiate into osteocytes. This process involves significant changes in cell morphology and gene expression.
Osteoblast to Osteocyte Transition
The transition from osteoblast to osteocyte involves the downregulation of genes associated with bone formation and the upregulation of genes specific to osteocytes. Key transcription factors such as Runx2 and Osterix are involved in this differentiation process. As osteoblasts become embedded in the matrix, they extend their cytoplasmic processes and establish connections with neighboring cells through gap junctions.
Maturation of Osteocytes
Once embedded in the matrix, osteocytes undergo further maturation, characterized by the development of an extensive canalicular network. This network is essential for the osteocytes' ability to sense mechanical signals and communicate with other bone cells. Mature osteocytes express specific markers such as dentin matrix protein 1 (DMP1) and sclerostin, which are involved in their regulatory functions.
Pathophysiology
Osteocytes are implicated in various bone diseases and conditions. Dysregulation of osteocyte function can lead to impaired bone remodeling and mineralization, contributing to skeletal disorders.
Osteoporosis
In osteoporosis, the balance between bone formation and resorption is disrupted, leading to decreased bone density and increased fracture risk. Osteocytes play a role in this process by regulating the activity of osteoblasts and osteoclasts. Reduced mechanosensation and increased apoptosis of osteocytes are observed in osteoporotic bones, contributing to the disease pathology.
Osteoarthritis
Osteoarthritis is a degenerative joint disease characterized by the breakdown of cartilage and changes in bone structure. Osteocytes in subchondral bone are involved in the pathological changes observed in osteoarthritis. Altered osteocyte signaling can lead to abnormal bone remodeling and sclerosis, exacerbating joint degeneration.
Osteogenesis Imperfecta
Osteogenesis imperfecta is a genetic disorder characterized by brittle bones and frequent fractures. Mutations affecting collagen production and osteocyte function are implicated in this condition. Osteocytes in osteogenesis imperfecta exhibit abnormal morphology and impaired mechanosensation, contributing to the fragility of bones.
Research and Clinical Implications
Osteocytes are a focus of extensive research due to their central role in bone biology and their involvement in various bone diseases. Understanding the molecular mechanisms underlying osteocyte function and signaling can lead to the development of novel therapeutic strategies for bone disorders.
Therapeutic Targets
Targeting osteocyte signaling pathways offers potential therapeutic approaches for bone diseases. For example, inhibiting sclerostin, a protein produced by osteocytes that inhibits bone formation, has shown promise in the treatment of osteoporosis. Sclerostin inhibitors such as romosozumab have been developed and are being evaluated in clinical trials.
Biomarkers
Osteocyte-derived molecules can serve as biomarkers for bone health and disease. For instance, elevated levels of sclerostin and DMP1 in the serum are associated with bone disorders such as osteoporosis and osteoarthritis. Monitoring these biomarkers can aid in the diagnosis and management of bone diseases.
Conclusion
Osteocytes are essential for the maintenance, repair, and metabolism of bone tissue. Their unique morphology and strategic positioning within the bone matrix enable them to sense mechanical signals and regulate bone remodeling. Dysregulation of osteocyte function is implicated in various bone diseases, making them a critical focus of research and therapeutic development. Understanding the complex biology of osteocytes is key to advancing the treatment of skeletal disorders and improving bone health.
